Table of Contents
Introduction
In the rapidly evolving landscape of pharmaceuticals, launching a new product is a critical endeavor, necessitating a strategic approach to ensure success. With advancements in healthcare occurring at an unprecedented rate, companies must adapt their strategies to maximize profitability and make a significant impact. This article explores the transformative potential of pre-launch analytics in the pharmaceutical industry and outlines essential steps for a successful product launch.
In the highly competitive pharmaceutical market, data-driven decision-making is paramount. We will delve into how leveraging pre-launch analytics can provide invaluable insights into the market landscape, enabling companies to refine their marketing, distribution, and pricing strategies. By employing methods such as the Google Ventures HEART framework and GAME framework tailored to the pharmaceutical industry, companies can optimize the customer experience and develop robust analytics strategys.
Furthermore, we’ll discuss the importance of incorporating industry benchmarks and product analytics solutions throughout the product development and design and development process. This approach ensures that the product meets the needs of both healthcare providers and patients while adhering to regulatory standards. Additionally, we’ll explore strategies for monetization, revenue generation, and advertising to enhance sales and drive success in the pharmaceutical market.
As personalized medicine and digital health solutions continue to reshape the industry, understanding and embracing these trends are crucial for the success of a pharmaceutical product launch. By adopting patient-centric approaches and staying abreast of regulatory changes, companies can position themselves for success in the ever-evolving pharmaceutical landscape. Join us on this journey to discover how to evolve your pharma product launch strategy and maximize profitability in this dynamic field.
Book a demo to experience the meaningful insights we derive from data through our launch analytical tools and platform capabilities. Schedule a demo today!
Request a Free DemoImportance of pre-launch analytics in the pharmaceutical industry:
In the fiercely competitive pharmaceutical landscape, a drug’s efficacy alone is no longer enough to guarantee success. It’s often said that a drug is only as good as its pre-launch strategy, and in today’s crowded market, this adage rings truer than ever. To stand out amidst the myriad of therapeutic options, pharmaceutical companies must meticulously craft strategies that differentiate their drugs across the entire value chain – from payers, and physicians, to patients.
This is where pre-launch analytics emerges as a game-changer. Leveraging product analytics solutions, pharmaceutical companies can harness insights from the customer journey and industry benchmarks to inform their product development process and design and development process. By employing analytics strategy such as the HEART framework and the GAME framework, companies can optimize their customer experience, ensuring that their drugs meet the needs and expectations of healthcare professionals and patients alike.
Moreover, pre-launch analytics enables companies to drive sales and revenue by informing effective monetization strategies and marketing campaigns. By analyzing data on ads performance and sales trends, companies can allocate resources more effectively and maximize their return on investment.
In essence, pre-launch analytics is not just about getting a drug to market faster; it’s about ensuring its success by making informed business strategies based on comprehensive data analysis. It’s the cornerstone of modern pharmaceutical marketing, driving profitability and ultimately improving patients’ lives by ensuring they have access to the treatments they need.
The challenges of pharmaceutical companies due to a lack of pre-launch analysis:
Drug acquisition and marketing, pivotal for advancing medicine, often face a myriad of hurdles that impede efficiency and pace. Firstly, a profound lack of transparency within the process obscures its inner workings, hindering ROAS assessment, resource allocation, and learning from past failures.
Secondly, siloed efforts across different research wings are a pervasive issue. Collaboration and information exchange between teams are often stifled, impeding the holistic utilization of expertise and data. This not only elongates timelines but also inhibits the cross-fertilization of ideas crucial for innovation. Thirdly, hunch-based scientific decisions dominate the landscape, introducing an element of uncertainty. Relying solely on expert intuition can be limiting, as it lacks the empirical foundation that analytics and metrics provide.
Moreover, the extreme dependency on subject matter experts can create bottlenecks. Limited access to data-driven support tools means that scientists may not have readily available resources to bolster their decision-making processes, further impeding progress.
In addressing these issues, a shift towards transparency, collaboration, data-driven insights, and the democratization of information can potentially revolutionize drug discovery, accelerating the development of life-changing treatments. Creating activation funnels, optimizing signup and conversion rates, enhancing engagement, and ensuring retention are vital steps towards achieving these goals. Integrated analytics dashboards and streamlined user pathways facilitate informed decision-making and drive sustainable growth in the pharmaceutical industry.
Experience the advantages firsthand by testing a customized complimentary pilot designed to address your specific capacity planning requirements. Pilot studies are non-committal in nature.
Request a Free PilotKey Benefits of pre-launch analytics in the pharmaceutical industry:
Pre-launch analytics in the pharmaceutical industry offer a myriad of benefits, crucial for optimizing product development and ensuring regulatory compliance. Leveraging various data sources, including A/B testing, surveys, and feedback tools, pharmaceutical companies can gain invaluable insights into user behavior, preferences, and industry trends. This enables the product team to identify optimization opportunities and refine user flows, ultimately enhancing the performance of their mobile product. Alerts and crash reports provide early detection of errors and performance roadblocks, enabling swift resolution by the product manager. Collaborative tools like shared dashboards and Countly Team facilitate seamless communication and project tracking, ensuring efficient project management. Furthermore, stringent adherence to data security, privacy regulations, and protocols such as GDPR, CPRA, and HIPAA ensures the protection of sensitive user information and regulatory compliance. By adopting pre-launch analytics, pharmaceutical companies can develop a robust Minimal Viable Product (MVP), garner positive ratings, and achieve success on the App Store while staying ahead of competitors.
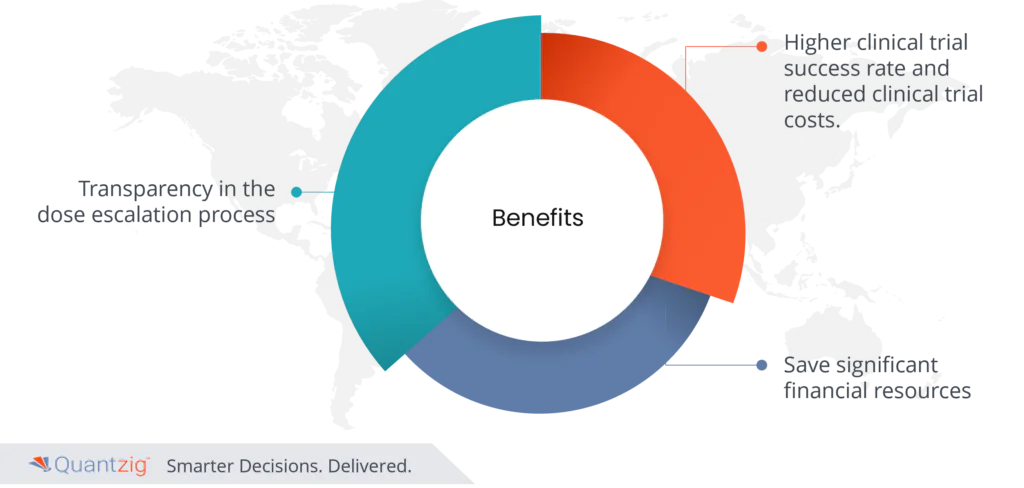
- Transparency in the dose escalation process
Pre-launch analytics in the pharmaceutical industry offers a transformative array of key benefits, and among them, transparency in the dose escalation process stands out as a critical advantage. Traditionally, this process has been plagued by uncertainty, relying heavily on subjective judgment. Pre-launch analytics revolutionizes this by introducing data-driven insights, providing a clear and objective view of a drug’s behavior at different dosage levels. This newfound transparency enhances decision-making, ensuring safer and more effective treatments for patients.
Furthermore, pre product launch through pre-launch analytics significantly contributes to cost reduction and efficiency. By identifying optimal patient populations, streamlining clinical trials, and predicting potential challenges, this process leads to higher clinical trial success rates and reduced costs. This empowers pharmaceutical companies to allocate resources more judiciously and accelerates the development timeline, ultimately getting life-saving medications to patients faster.
In essence, pre-launch analytics reshapes the pharmaceutical landscape, offering a strategic edge by promoting transparency, improving cost-effectiveness, and expediting the availability of innovative therapies, ultimately benefiting both the industry and the patients it serves.
- Higher clinical trial success rate and reduced clinical trial costs.
Pre-launch analytics represents a revolutionary paradigm shift in the pharmaceutical industry, delivering a host of pivotal benefits. Among these, achieving a higher clinical trial success rate and reduced clinical trial costs emerge as paramount advantages. It harnesses the power of data-driven insights to optimize the entire drug development process. It enables pharmaceutical companies to identify the most promising drug candidates, optimize trial designs, and pinpoint ideal patient populations. This precision leads to higher success rates, as fewer resources are wasted on unsuccessful trials. Moreover, it empowers companies to predict potential obstacles and adapt strategies, accordingly, mitigating risks and enhancing trial efficiency. By avoiding costly missteps and expediting the path to regulatory approval, it drastically cuts clinical trial costs.
Ultimately, product pre-launch ideas with analytics not only saves significant financial resources but also accelerates the availability of life-saving medications, benefiting both pharmaceutical companies and patients. It embodies a transformative approach that promises to revolutionize drug development, making it more efficient, cost-effective, and patient-centric. Leveraging tools such as Google Ventures, the HEART framework, and the GAME framework can enhance the effectiveness of product analytics solutions, optimizing the entire product development process. By incorporating industry benchmarks and analytics strategy into business strategies, companies can better understand the customer journey and improve the overall customer experience. Monetization and revenue generation can be further optimized through insightful analysis of ads performance within the design and development process.
Get started with your complimentary trial today and delve into our platform without any obligations. Explore our wide range of customized, consumption driven analytical solutions services built across the analytical maturity levels.
Start your Free TrialConclusion:
In conclusion, the pharmaceutical industry is at a pivotal juncture, where the integration of pre-launch analytics and a strategic product launch approach can reshape its future. By harnessing the power of data-driven decision-making, optimizing launch strategies, and adapting to current industry trends, pharmaceutical companies can unlock new avenues for profitability. The steps involved in product launch, from pre-launch analytics to market adaptation, form the backbone of this transformation. Embracing patient-centric approaches, personalized medicine, and technological advancements, the industry is poised for remarkable growth. As the pharmaceutical landscape evolves, those who embrace innovation and strategic adaptability will not only thrive but also contribute to the advancement of global healthcare.
In this transformative journey, tools and frameworks such as Google Ventures, the HEART framework, and the GAME framework play crucial roles. These frameworks provide structured approaches to decision-making, ensuring that product development processes are aligned with customer needs and industry benchmarks. Moreover, strategies for monetization and revenue generation, including ads, are essential considerations in shaping business strategies and ensuring sustainable growth. Leveraging product analytics solutions and an analytics strategy enables companies to understand the customer journey, enhance customer experience, and make informed decisions throughout the design and development process. As the industry progresses, the convergence of these elements, alongside industry benchmarks, will be instrumental in driving innovation and competitiveness.
Success Story:
Pioneering Success: Revolutionizing Pharmaceutical Pre-launch Analytics
Client Details: A prominent player in the pharmaceutical industry located in the United States, faced a daunting challenge with an upcoming product launch. They needed to optimize their pre-product launch strategy to ensure a successful market entry in a highly competitive landscape.
Challenges:
The client faced a multitude of formidable challenges in their pursuit of first-to-market advantage within the rare disease area of drug discovery. Foremost among these hurdles was the prolonged drug discovery timeline, significantly delaying their ability to bring a potentially life-saving treatment to patients in need. This delay was exacerbated by the lack of clarity surrounding the outcome timelines, making it challenging to set realistic expectations and allocate resources effectively. Additionally, the extended drug discovery phase was driving up operational costs, straining the financial resources required to sustain research and development efforts. This financial burden was compounded by the uncertainty of success, as the client could not ascertain when, or if, their drug candidate would reach the market.
In such a competitive and high-stakes environment, overcoming these challenges demanded a multifaceted approach, encompassing innovation, data-driven decision-making, and strategic partnerships to expedite drug discovery and enhance the likelihood of achieving that coveted first-to-market status.
Solutions:
Harnessing the power of edge computing techniques, our collaboration with a prominent pharmaceutical leader marked a groundbreaking advancement in the realm of drug discovery. By employing edge computing in tandem with bioinformatics, we achieved remarkable feats.
First and foremost, we facilitated the rapid identification of the crystal structure of a virus, a critical step in understanding its vulnerabilities and potential drug targets. This breakthrough significantly expedited the early stages of drug discovery. Furthermore, our partnership enabled the prediction of suitable antigens, an essential component in the development of vaccines and therapeutic antibodies. Leveraging bioinformatics and edge computing, we identified the most promising candidates for further research and development.
In addition, we employed data-driven approaches to identify potential drug compounds, streamlining the search for effective treatments. Our computational prowess accelerated the screening process, saving both time and resources.
Finally, our analytical capabilities were instrumental in fine-tuning the drug discovery process by meticulously analyzing trial results. This data-driven approach enabled our client to make informed decisions, optimizing their drug development strategy.
Overall, the integration of edge computing, bioinformatics, and data-driven analysis represents a transformative leap forward in pharmaceutical research. It not only expedites the drug discovery timeline but also enhances the precision and efficacy of the process, ultimately bringing life-saving medications to patients more swiftly and effectively.
Impact Delivered:
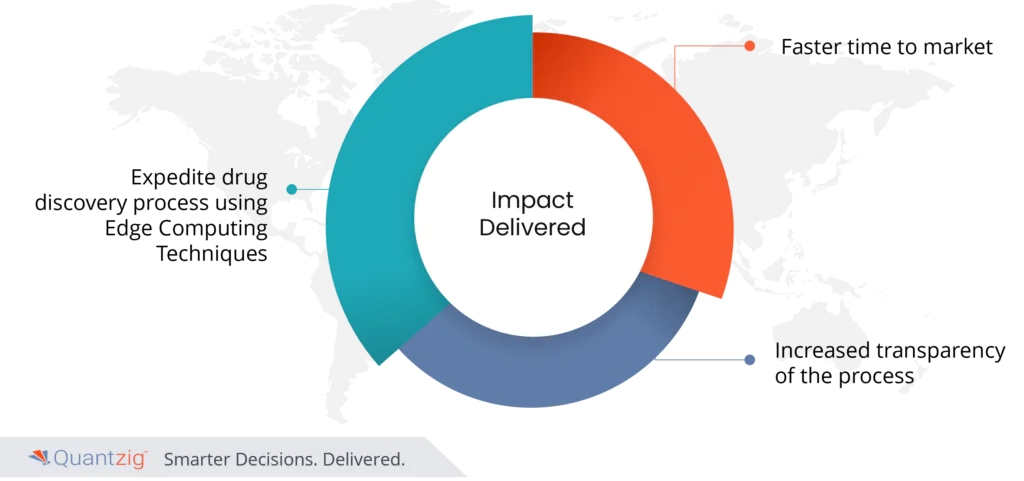
- Expedite drug discovery process using Edge Computing Techniques
- Faster time to market
- Increased transparency of the process
Ready to unlock the potential of pre-launch analytics and revolutionize your pharmaceutical success? Contact Quantzig today to discover how our data-driven solutions can accelerate your product launch and drive profitability in this dynamic industry.