Table of Contents
Introduction to Clinical Data Management
Clinical Data Management (CDM) is a critical function in clinical research, ensuring that the data generated from trials is accurate, reliable, and compliant with regulatory standards. With the advent of digital transformation, CDM has significantly evolved, integrating advanced technologies such as Artificial Intelligence (AI), Machine Learning (ML), blockchain, and real-time analytics. These innovations enhance data processing efficiency, minimize errors, and improve the overall quality of clinical trial data.
As clinical trials become increasingly complex—with multi-site studies, decentralized trial models, and diverse data sources—effective CDM strategies are essential for maintaining data integrity, patient safety, and regulatory compliance. From managing vast data sets to ensuring data security and regulatory compliance, clinical data management teams must navigate a complex landscape of technological, operational, and organizational obstacles.
This article explores the top challenges in clinical data management and discusses strategic approaches that organizations can adopt to overcome these obstacles and optimize their data management practices.
Book a demo to experience the meaningful insights we derive from data through our analytical tools and platform capabilities.
Request a DemoWhat is Clinical Data Management (CDM)?
Clinical data management (CDM) is the process of collecting, organizing, validating, and analyzing data generated during clinical trials and healthcare research activities. The primary goal of CDM is to provide high-quality, reliable, and statistically sound data by minimizing errors and missing data and gathering the maximum amount of data for analysis. CDM involves various activities such as case report form (CRF) design, database development, data entry, data cleaning, medical coding, and database locking.
Why is CDM Important?
CDM is critical for the success of clinical trials and healthcare research as it ensures the integrity, accuracy, and completeness of the data collected. High-quality data obtained through rigorous CDM processes is essential for regulatory submissions, data analysis, and drawing valid conclusions about the safety and efficacy of new medical products. Effective CDM also helps accelerate the drug development process by facilitating faster data processing and decision-making.
What are the Top Challenges in Clinical Data Management System?
Today, all modern industries and companies are embracing the power of digital technologies. The healthcare industry is also following the trend by using patient data management systems, which keep track of patient information and medical history thereby replacing handwritten medical files. Since patient data is sensitive, medical companies and professionals should adopt new strategies to manage such healthcare data securely. Clinical data management simplifies a lot of operational tasks thereby increases the efficiency of healthcare providers. However, it can be challenging to manage such large and complex sets of healthcare data. Clinical data management is further complicated by various government regulations and compliance issues.
HIPAA Compliance
The Health Insurance Portability and Accountability Act (HIPAA) mandates strict security protocols for sharing Electronic Health Records (EHRs) among practitioners and patients. Healthcare providers face the dual challenge of maintaining closed-network security while enabling data accessibility. Data breaches in this context carry severe financial and reputational risks, requiring robust safeguards to balance compliance with operational needs.
Sharing Patient Data
Regulatory constraints often limit how patient data is shared between medical professionals, creating ambiguity in collaboration. While solutions like EHRs and cloud computing aim to streamline data exchange, standardizing platforms across institutions remains a hurdle. Inconsistent access protocols and integration challenges further complicate secure, efficient data sharing industry-wide.
Mobile Computing
The demand for mobile access to clinical data clashes with the need for stringent security in healthcare environments. Transitioning to mobile platforms requires wireless infrastructure that meets compliance standards, alongside new protocols to protect sensitive data on devices. Ensuring seamless, secure access across care facilities remains a critical operational challenge.
Operational Analytics
While EHRs have improved care quality and efficiency, traditional metrics like patient care data inadequately measure workforce performance. Healthcare managers now seek advanced analytics strategies to mine data for productivity insights, profitability analysis, and actionable performance indicators that better identify areas for improvement.
Data Volume and Complexity
The exponential growth of clinical data strains Clinical Data Management (CDM) systems, causing delays in processing and reporting. Diverse data formats, terminologies, and standards add layers of complexity, making it difficult to maintain data quality. Effective management demands scalable systems capable of handling both volume and variability.
Data Quality and Integrity
Inaccuracies from manual entry, inconsistent terminology, and non-standardized formats undermine data reliability. Migration and integration processes further risk data integrity. Rigorous validation, verification, and quality control processes are essential to ensure accuracy and consistency in clinical datasets.
Data Security and Privacy
Rising cyber threats, such as breaches and ransomware, necessitate robust protection of digital health records. CDM systems must enforce encryption, access controls, and audit trails to secure data storage and transmission. Compliance with privacy regulations remains a priority to prevent unauthorized access and maintain patient trust.
Interoperability
Integrating disparate systems like EHRs and clinical trial management systems (CTMS) is critical for seamless data exchange. Achieving interoperability requires standardized formats, data mapping, and system compatibility, which are often hindered by fragmented technologies. Overcoming these barriers is key to enabling cohesive communication across the clinical ecosystem.
Also Read: Pharma Customer Segmentation Helped a Client Improve Customer Satisfaction by 10%
Experience the advantages firsthand by testing a customized complimentary pilot designed to address your specific requirements. Pilot studies are non-committal in nature.
Request a Free PilotWhat are the Strategies to Overcome the Clinical Data Management System Challenges?
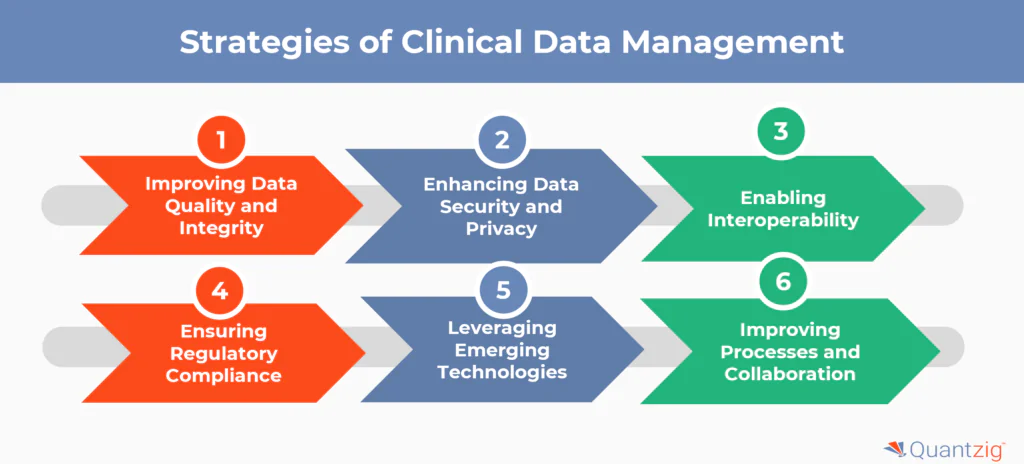
Some key strategies to overcome the challenges in clinical data management system include:
1. Improving Data Quality and Integrity
- Implement robust data validation checks and automated validation protocols to ensure data accuracy
- Conduct regular audits and employ statistical methods for anomaly detection to maintain data integrity
- Standardize data formats and terminology to reduce complexities
- Provide comprehensive training to data entry personnel on proper data entry techniques
2. Enhancing Data Security and Privacy
- Employ advanced encrypted technologies and stringent access controls to protect sensitive patient data
- Regularly conduct statistical risk assessments and audits to identify vulnerabilities and address potential security threats
- Ensure compliance with data privacy regulations like HIPAA and GDPR
- Implement robust backup and disaster recovery plans to safeguard against data loss
3. Enabling Interoperability
- Adopt common data standards and terminologies like CDISC to facilitate data exchange
- Develop APIs and middleware to integrate diverse systems and data sources
- Participate in industry initiatives for interoperability like the CDISC Interchange
4. Ensuring Regulatory Compliance
- Stay informed about evolving regulations through industry associations and regulatory bodies
- Conduct statistical analysis to assess the impact of regulatory changes and ensure timely adjustments
- Maintain thorough documentation and audit trails for inspections and audits
- Implement quality management systems to proactively identify and mitigate compliance risks
5. Leveraging Emerging Technologies
- Utilize AI and machine learning for data cleaning, anomaly detection, and predictive modeling
- Adopt cloud computing for scalable data storage, processing, and collaboration
- Explore blockchain technology for secure, decentralized data sharing and provenance
- Implement natural language processing (NLP) to extract insights from unstructured data like clinical notes
6. Improving Processes and Collaboration
- Adopt agile methodologies for faster, iterative development and deployment of CDM systems
- Involve stakeholders like investigators, sponsors, and CROs in process re-engineering
- Streamline workflows and eliminate redundancies to improve efficiency
- Foster cross-functional collaboration and communication to align on goals and priorities
By strategically addressing these challenges through a combination of process improvements, technology adoption, and collaborative approaches, organizations can enhance the quality, efficiency, and impact of their clinical data management practices.
Also Read: Customer Loyalty Analysis for a Leading Pharma Player in Europe
Get started with your complimentary trial today and delve into our platform without any obligations. Explore our wide range of customized, consumption driven analytical solutions services built across the analytical maturity levels.
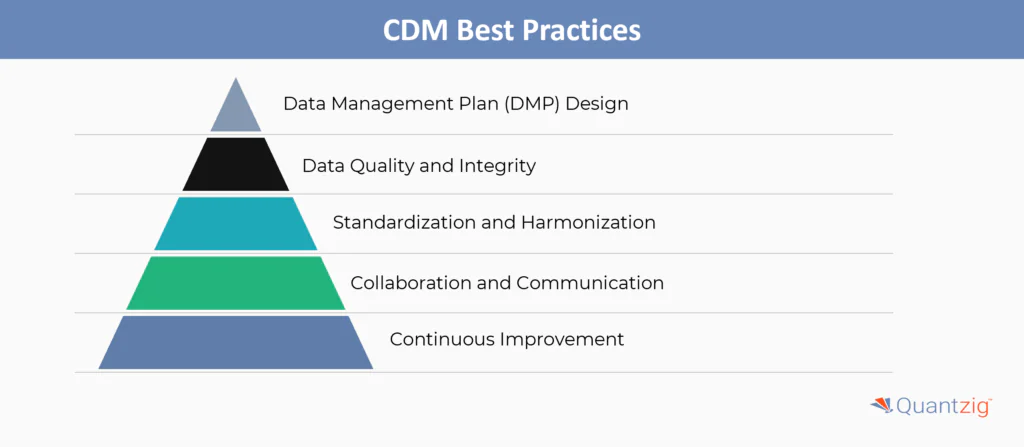
Start your Trial TodayData Management Plan (DMP) Design
A robust Data Management Plan (DMP) is foundational, detailing procedures for data collection, storage, validation, and governance. Alignment with regulations (e.g., HIPAA) and standards like CDASH and SDTMIG ensures compliance and interoperability. The DMP must evolve dynamically, adapting to study changes or emerging requirements to maintain relevance throughout the trial lifecycle.
Data Quality and Integrity
High-quality data is ensured through automated validation checks, manual reviews, and quality control protocols. Audit trails document all data modifications, ensuring traceability and accountability. Compliance with 21 CFR Part 11 safeguards electronic records and signatures, reinforcing data integrity for regulatory acceptance.
Standardization and Harmonization
Adopting standardized formats (e.g., CDISC SDTM) and terminologies (e.g., MedDRA, WHO Drug) minimizes variability across studies. Harmonizing data structures streamlines regulatory submissions and cross-study analysis, reducing errors and accelerating timelines.
Collaboration and Communication
Effective CDM requires alignment among sponsors, investigators, and data managers. Clear communication channels and roles ensure transparency, while regular updates and risk reports keep stakeholders informed and proactive in addressing issues.
Continuous Improvement
Regularly auditing processes and incorporating feedback drives efficiency. Training programs keep teams updated on industry advancements, while benchmarking against best practices ensures CDM workflows remain agile, scalable, and future-ready.
Conclusion
As clinical trials grow more complex, efficient Clinical Data Management (CDM) becomes essential for ensuring data integrity, regulatory compliance, and accelerated drug development. The integration of AI, decentralized trial models, biostatistics, and risk-based monitoring is revolutionizing CDM, making it more efficient, accurate, and adaptable.
Organizations that invest in advanced CDM technologies, strong governance frameworks, and AI-driven automation will be well-positioned to navigate the evolving landscape of clinical research and drive faster, more reliable trial outcomes.